Environment & Energy
Related: About this forumPesky Thermodynamics: The Mathematics of Wasting Energy by Storage in Li Batteries.
The paper I'll discuss in this post is this one: G. Richardson, I. Korotkin, Heat generation and a conservation law for chemical energy in Li-ion batteries, Electrochimica Acta, Volume 392, 2021, 138909,
All systems that convert energy from one form to another waste energy. This is why, despite endless rhetoric claiming that energy storage is "green," under the mistaken impression, that so called "renewable energy" is available in huge excesses and we should be storing it, energy storage is making things worse, faster, not better, as I reported here earlier today as I do every spring:
New Weekly CO2 Concentration Record Set at the Mauna Loa Observatory, 430.86 ppm
The trillion dollar reactionary fantasy about making our energy supplies dependent on the weather is a miserable failure, but it has inspired dangerous and, frankly, stupid, "investment" in energy storage, in one case hydrogen, in another, batteries. So called "renewable energy" is useless if the point is to address the extreme global heating now accelerating. It has done nothing other than to entrench and expand the use of dangerous fossil fuels, the waste of which, chiefly CO2, although there are other forms of fossil fuel waste that are more immediately toxic, is widely distributed throughout the planet.
Batteries are powered overwhelmingly by fossil fuels as a primary energy source; this is true if the batteries are in a car, a computer, a cell phone or any other device. Hydrogen, whether electrolytic (which is enormously expensive and trivial), or more commonly and less expensively made by steam reforming, generally of natural gas or coal, or as a side product of petroleum refining, is overwhelmingly made from fossil fuels. To state otherwise is either to lie or to be joking.
For the reasons given above, the fantasy that electric cars are "green" is only nominally true of electricity is carbon free, which, in most places, including so called "renewable energy" wonderlands, it is not. Except for places where riparian and riverine ecosystems have been destroyed by dams, or nations like France, this is not generally true. The lack of reliability for the expensive land and mass intensive wind and solar industries, again, to repeat, merely entrenches reliance on fossil fuels, usually gas, but in places like Germany, coal as well.
The laws of thermodynamics are pretty clear for the largest, by far, industrial energy transformation on the planet, the combustion of fossil fuels to make mechanical energy that can be further modified to make electricity, the very common form of energy that is nonetheless greatly thermodynamically degraded with respect to the dangerous fossil fuels from which it is overwhelmingly made.
Conversely, the storage of electrical energy in batteries, the conversion of electricity back to chemical energy, degrades the thermodynamics of energy further but by how much is highly variable and, in fact, highly complex. That degradation happens should be obvious to anyone who has touched a battery being charged or discharged. They release heat, sometimes so much heat that they can, in the case of lithium batteries, burst into flame. (On an industrial grid scale, this recently happened at a battery facility in Morro Bay California, spewing some unpleasant toxic residue all over that beautiful area.)
The complexity can be realized by taking a peak at the paper cited at the outset of this post.
Some text from the paper:
The Doyle Fuller Newman (DFN) model [1], [5], [12], [13], [23], which is also often called the Newman model or the P2D model, has proved itself to be an extremely useful, and versatile, tool for understanding Li-ion battery performance. Recent works have shown that, providing that lithium ion transport within the electrode particles is modelled by an appropriately calibrated nonlinear diffusion equation, the model is capable of accurately predicting battery performance [7], even when subjected to highly non-uniform drive cycles [38]. While these predictions of the DFN model provide an accurate relation between the cell voltage and the current draw they have not, to date, been used to provide a consistent picture of the irreversible energy losses occurring within the cell. While there are many works that use DFN to estimate irreversible energy loss and heating within Li-ion cells none of them uses a theory of energy dissipation that is consistent with the DFN model. In this context we note the following works that are based on DFN simulation [4], [6], [9], [10], [14], [16], [18], [19], [22], [24], [25], [31], [32], [33], [35] and [2], which is based on a single particle model (a simplification of the DFN model). All of these works predict energy losses without accounting for the enthalpy of mixing (or heat of mixing) in the electrode particles and only partially account for the irreversible energy loss in the electrolyte, again neglecting the enthalpy of mixing. This method of estimating the energy dissipation is based on thermodynamic treatments by Rao and Newman [27] and Gu and Wang [15], which are ultimately based on the work of Bernardi [3]. We note also the works Tranter et al. [34] and Farag et al. [11], which are both based on the DFN model, but use alternative methods for estimating heat production, neither of which are consistent with overall energy conservation within the DFN model, although Farag et al. do approximate the heat of mixing within the electrode particles, noting that it is often significant. Finally, we remark that Latz and Zausch [20], [21] have used a thermodynamic method to estimate energy dissipation in a lithium cell and, as we shall show, obtain expressions for irreversible energy losses within the device that are consistent with the energy conservation law that we derive here from the DFN model. However, as far as we are aware, their work has never been applied to the DFN model. The relative lack of attention that their estimate of energy dissipation has received can be attributed to (I) the large number of other works in the literature that use thermodynamic arguments to arrive at incomplete estimates of the irreversible energy losses and (II) the fact that there is no independent theoretical procedure for determining which of these thermodynamic approaches are correct. The current work aims to address the second of these points and thereby fill a significant void in the literature.
Given the widespread use, and overall utility, of the DFN model of Li-ion battery behaviour it would be a major step forward to unequivocally establish the form of the irreversible energy dissipation law that is consistent with this model. This will not only allow accurate modelling of heat generation in composite cells (such as cylindrical and pouch cells), in which inadequate cooling can lead to significant temperature heterogeneities, but could also be used as a design tool in order to identify the components of the cell in which irreversible losses are most significant under the cell’s characteristic operational conditions. To date, a unifying theory of energy transport and dissipation in the DFN model remains to be established and it is precisely this omission that we aim to address here.
I won't have much time to go into the significant detail of the paper, but as an indicator of the complexity of the problem, let me share graphics of some of the tables that go into the topic.
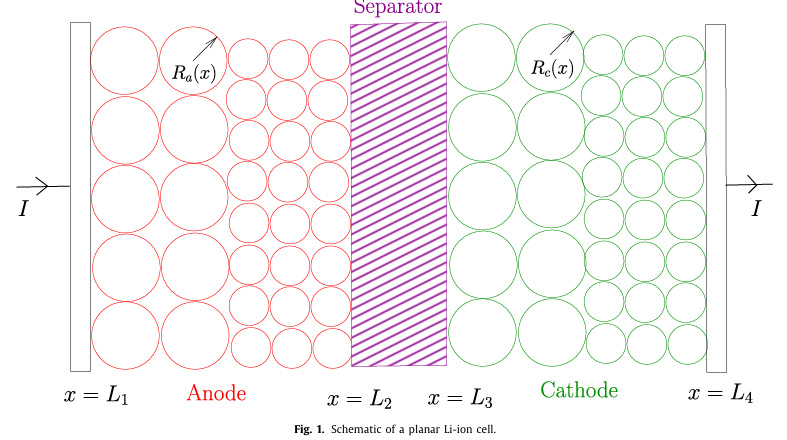
A figure from the paper referring to regions in a battery to which the variables and equations will apply:
Tables of variables and of functions utilized in the paper:
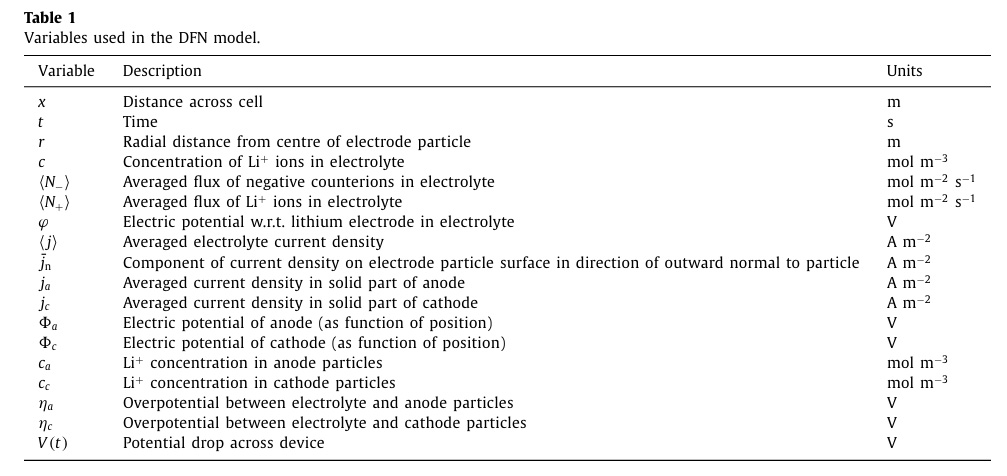
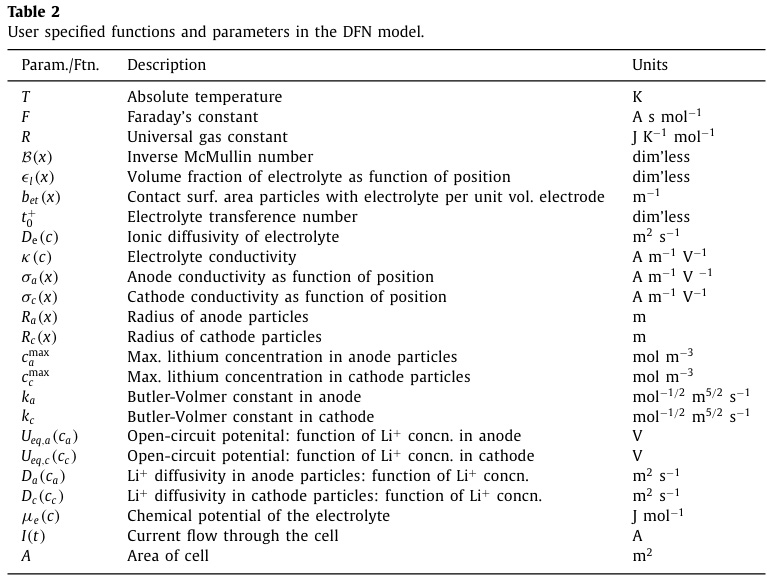
A minor quibble is the inclusion of temperature as a function; to my mind it's an intensive variable.
The following equation refers to all the ways that energy can be lost to heat, designated Q with subscripts and superscripts.
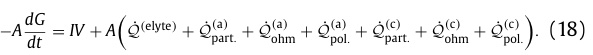
Equations for the various heat dissipation energy losses:
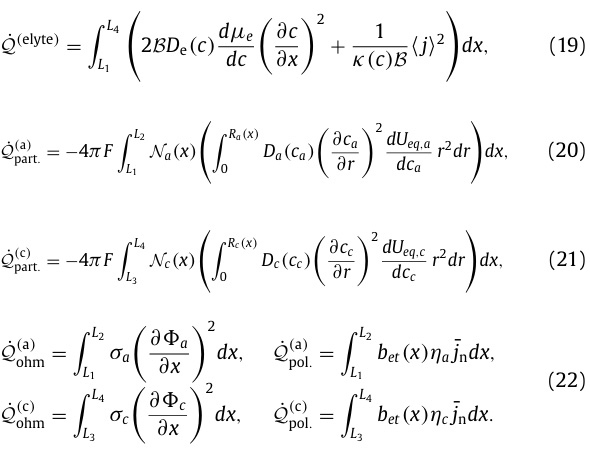
The limits of integration refer to the distances in the figure.
Engineers, work to design batteries that minimize these losses, but the prevention of losses is impossible. The laws of thermodynamics are not invalidated by wishful thinking.
Have a nice work week if you are working.
Bernardo de La Paz
(60,320 posts)That doesn't make nuclear generated hydrogen a waste, since it can be generated by electrolysis with no fossil fuel involved.
Some amount of storage is useful enough to be worth the cost of conversion and wastage in storage.
The goal should be to make non-fossil energy abundant enough that it can economically replace all fossil hydrocarbon usages except perhaps a reduced amount of plastic, reduced by recycling and replacing usages with more durable metals or fibre like carbon fibre for longer terms and plant fibre for "disposable" uses (even then with some recycling).
NNadir
(37,602 posts)I don't know how many times I have to say this, but there is a huge difference between "can" and "is."
Almost no hydrogen on this planet is made by electrolysis using nuclear generated electricity. Every nuclear plant on this planet makes electricity to prevent the use of fossil fuels for the same purpose, making electricity.
That's true in 2025, and it will remain true for the foreseeable future.
Bernardo de La Paz
(60,320 posts)There are two goals.
Reducing current emissions of CO2 and methane.
Sustainable energy without CO2 and methane.
The first leads to the second but the second is necessary to supersede the first. Also, the perfect is the enemy of the good. We won't get to perfect in one sudden giant step. There is no single technique in the short and medium run to get get closer to the perfection of long term sustainability.
NNadir
(37,602 posts)This is because electricity is already thermodynamically degraded.
It is possible, the Chinese are apparently piloting this, to make nuclear thermochemical hydrogen for industrial use, but hydrogen itself, even under these more efficient processes is thermodynamically degraded, no matter how many morons from the fossil fuel industry post slick ads here with "green hydrogen" bullshit to greenwash fossil fuels. That would make hydrogen relatively cleaner, but it will still involve energy losses.
What the fuck is the source of all this hydrogen mysticism? How many decades to we have to listen to this chanting nonsense? The gas has terrible physical properties, an exceedingly low critical temperature, the third lowest of any gas, with only 4 being lower He 3He, has a positive Joule Thompson coefficient, low explosive limits, incompatibility with many metals, and extremely low viscosity. What is it exactly? That we all learn in high school that the product of hydrogen combustion is water? The combustion product of methane is water as well (along with carbon dioxide).
It's a useful captive intermediate to make ammonia and methanol, but it isn't green and won't ever be green, as the laws of thermodynamics are inviolable.
