Potentially Prebiotic Synthesis of Aminoacyl-RNA via a Bridging Phosphoramidate-Ester Intermediate
The paper to which I'll briefly refer is this one: Potentially Prebiotic Synthesis of Aminoacyl-RNA via a Bridging Phosphoramidate-Ester Intermediate Samuel J. Roberts, Ziwei Liu, and John D. Sutherland, Journal of the American Chemical Society 2022 144 (9), 4254-4259.
This is "RNA week" in my head, and as a result I came across this interesting paper not relevant to my work but interesting because of my interest in the origins of life, and in particular, the related topic of "origins of chirality" - the functional chemical asymmetry on which life depends.
The discovery that RNA can function in a role that is mostly involved in modern life forms with proteins, that is catalysis, has led to the development of theories of a putative "RNA world" in the prebiotic space, that is that the first molecules involved in the evolution of life were nucleobases conjugated to sugars, in modern RNA the sugar is ribose.
The simplest sugar, glyceraldehyde, is found in interstellar space in icy clouds.
(cf. Cruz-Castañeda, J., Aguilar-Ovando, E., Buhse, T. et al. The importance of glyceraldehyde radiolysis in chemical evolution. J Radioanal Nucl Chem 311, 1135–1141 (2017) and Jes K. Jørgensen, Cécile Favre, Suzanne E. Bisschop, Tyler L. Bourke, Ewine F. van Dishoeck, and Markus Schmalzl,DETECTION OF THE SIMPLEST SUGAR, GLYCOLALDEHYDE, IN A SOLAR-TYPE PROTOSTAR WITH ALMA The Astrophysical Journal Letters, Volume 757 Number 1, 2012 L4)
It is not clear that this molecule is present in an enantiomeric excess in these clouds, but one hypothesis for the origin of chirality is asymmetric radiation in deep space: JOHN R. CRONIN AND SANDRA PIZZARELLO SCIENCE 14 Feb 1997 Vol 275, Issue 5302
pp. 951-955 Enantiomeric Excesses in Meteoritic Amino Acids
What is interesting in the paper described at the outset of this post is an indication of possible chiral amplification, suggesting, as is well known in the laboratory, a situation in which a chiral molecule reacts in such a way as to induce (or at least change the kinetics) involving other molecules in the system.
From the text:
Ribosomal peptide synthesis relies upon aminoacyl-tRNAs as intermediates in the translation of messenger-RNAs into the corresponding genetically coded protein products. Highly specific enzymatic aminoacylation of the 2′,3′-diol of tRNAs using aminoacyl-adenylates is crucial to this process. In the absence of enzymes, which bind them tightly and protectively, carbon dioxide promotes the reversible conversion of aminoacyl-adenylates to amino acid N-carboxyanhydrides (NCAs), and the latter are subsequently polymerized to random peptides in aqueous solution. (1,2) Other aminoacyl-RNA mixed anhydrides 1 have been used as substrates for non-enzymatic aminoacyl-RNA synthesis in prebiotic model studies, (3,4) but they are similarly susceptible to carbon dioxide-promoted destruction. Conceptually, there are two fundamentally different chemical pathways for the formation of mixed anhydrides─via either carboxylate activation or phosphate activation (the latter of which is used by modern day biology). In the former, the phosphate reacts (reversibly) with an activated carboxylate (e.g., NCA, 5(4H)-oxazolone, or other species) giving a mixed anhydride 1. (5−7) Conversely, in the phosphate activation pathway, a native amino acid reacts with an activated phosphate (e.g., imidoyl phosphate) giving a mixed anhydride 1. (7) Carboxylate activation processes (5,6) are inherently problematic under a carbon dioxide-containing atmosphere in that the formation of NCAs and hence random non-coded peptides cannot be avoided.
I have personally made NCA's in the lab, albeit not with mixed amino acids, but I have, although I wished to avoid doing so, made polymers of single amino acids.
The introduction continues:
Prebiotic activation chemistry is also needed to enable the formation of phosphodiesters from alcohols and the corresponding phosphomonoesters. Thus, for example, 5′-phosphorimidazolides are used for prebiotic RNA ligation or monomer extension chemistry. (8,9) Seeking to unify the various building block activation chemistries, we were intrigued by the work of Weber and Orgel et al. showing that amino acids can react with 5′-phosphorimidazolides through their amino groups generating amino acid phosphoramidates 2 (Scheme 1). (10) By using adenine nucleotide 2′/3′-aminoacyl-esters and 5′-phosphorimidazolides, bridged phosphoramidate-esters could be formed by templating with poly(U). (11) The Richert group has built upon these earlier results and showed that RNA-peptide phosphoramidates can couple to 3′-amino-2′,3′-dideoxy-terminated oligonucleotides to give bridging peptide phosphoramidate-amides on an RNA template. (12) However, the reaction of RNA-amino acid phosphoramidates 2 (Scheme 1) with the 2′,3′-diol of a canonical oligonucleotide 3 to generate phosphoramidate-esters 4 is inherently more interesting as the products are potentially hydrolyzable to aminoacyl-esters 5 and 5′-phosphoryl-RNAs 6 under mildly acidic conditions. (13) Synthesis of aminoacyl-esters in this way would bypass the unstable mixed anhydrides 1 employed in other strategies (14) and might have been more easily achievable before the advent of enzymes...
Scheme 1:
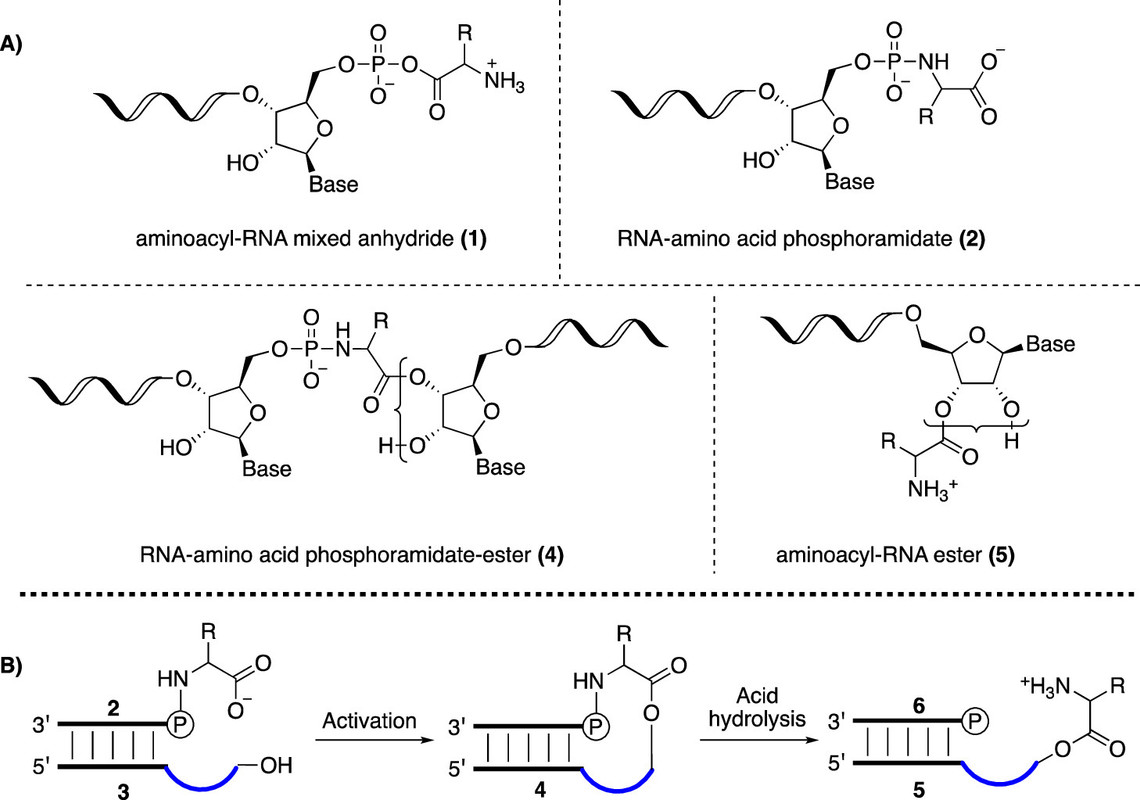
The caption:
Scheme 1. (A) Chemical Structures of RNA-Amino Acid Hybrid Compounds; (B) Reaction Scheme for the Formation of Aminoacyl-RNA Esters a
aRNA-amino acid phosphoramidate-ester (middle structure, 4) is formed from RNA phosphoramidate (2, left) and ester acceptor RNA (3) under activation conditions. The phosphoramidate bond can then be cleaved under mild acid conditions generating aminoacyl-RNA (5, right).
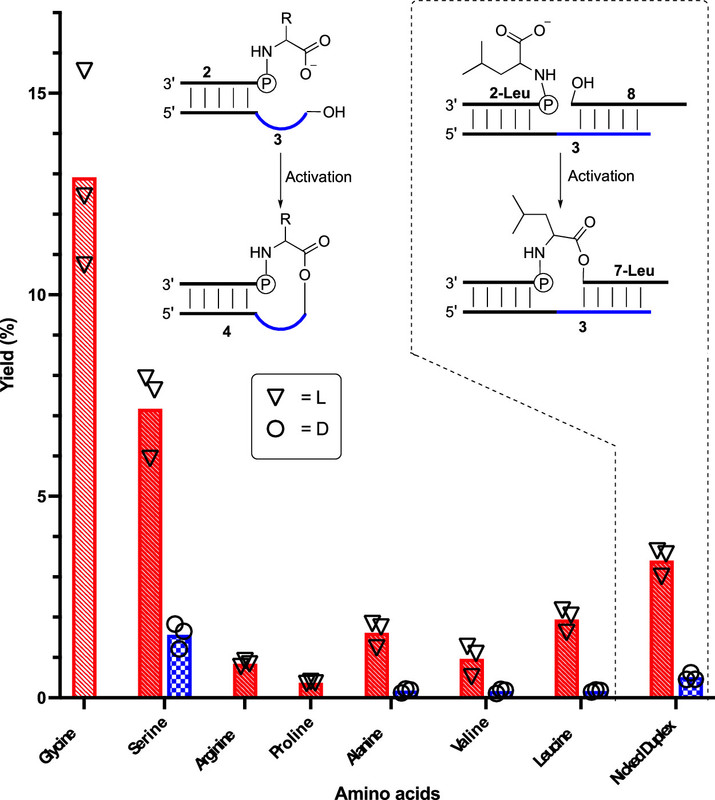
The caption:
Figure 1. Room-temperature yields for formation of phosphoramidate-esters 4 via a nicked loop and 7-Leu via a nicked duplex. Bars are mean values based on three replicates. Each replicate is represented by a circular/triangular data point. Red hashed bars represent l-amino acid stereochemistry and blue checkered bars d- amino acid (where appropriate).
The part I have put in bold shows that there is chiral amplification, one of the two enantiomers (molecules that are three dimensional mirror images) in the racemic pair (a system having equal amounts of both of the mirror image molecules) reacts more readily with a chirally pure molecule, in this case the nucleotide, faster and more readily.
In a sense, this means, were this mechanism prebiotic, it would partition the two enantiomers, allowing through any number of means, for their separation. It's a big deal to my mind.
Other figures from the paper:
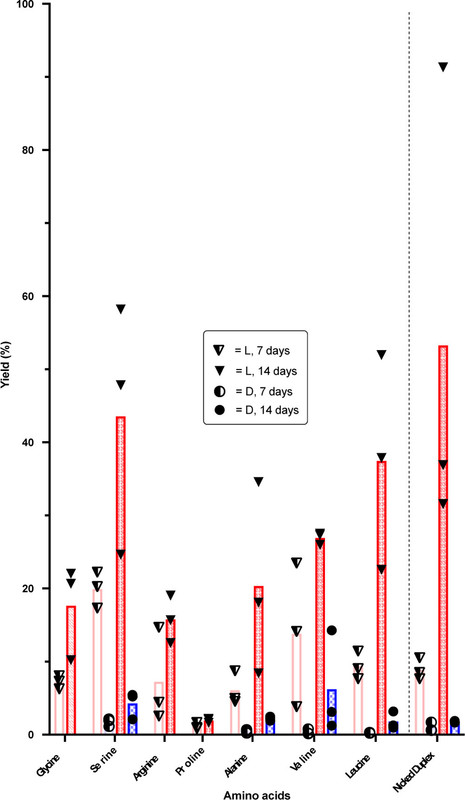
The caption:
Figure 2. Eutectic yields for formation of phosphoramidate-esters 4 via a nicked loop and 7-Leu via a nicked duplex. Each replicate is represented by a circular/triangular data point. Red hashed bars represent l-amino acid stereochemistry and blue checkered bars d-amino acid (where appropriate). Darker colors indicate longer reaction times.
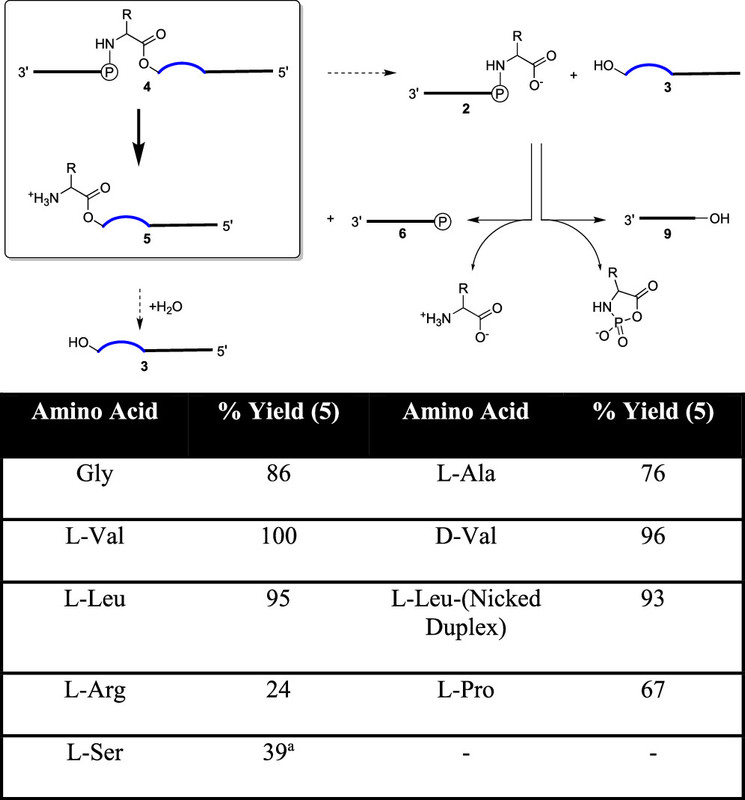
The caption:
Figure 3. Top─Mechanism of hydrolysis of the phosphoramidate-ester 4 at pH = 3. The majority of 4 undergo phosphoramidate bond hydrolysis giving 5′-phosphoryl-5-mer 6 and the desired aminoacyl-10-mer 5 product, which can then degrade slowly back to template 3. Minority hydrolyses at the ester bond of 4 gives 3 and 5′ phosphoramidate-5-mer 2. Phosphoramidate 2 degrades either by direct hydrolysis to 5′-phosphoryl-5-mer 6, or by intramolecular oligonucleotide dephosphorylation to the 5′-hydroxyl-5-mer 9 in an amino acid-specific fashion. Bottom─Yields of formation of aminoacyl-10-mer 5 from phosphoramidate-ester 4 with different amino acids. aEstimate due to unknown products of hydrolysis. A discussion of the differing yields of Arg, Pro, and Ser is presented in the ESI.
An excerpt of the paper's conclusions, mentioning the chiral enhancement:
This work has demonstrated that efficient production of aminoacyl-RNAs 5 can be achieved via stepwise esterification of RNA-amino acid phosphoramidates 2 to proximal oligonucleotide-2′,3′-diols 3, followed by mild acid hydrolysis of the phosphoramidate linkage. The lack of acidic degradation could be attributed to the short length of treatment (17 h), a factor which would have to be incorporated into the prebiotic scenario. The proximity required to enable esterification can be realized in nicked-loop or nicked-duplex configurations. The esterification reaction is stereoselective─being preferred for l- over d-amino acid residues with RNA based on d-ribose─and the stereoselectivity is highest in the nicked-loop configuration. The reaction with one set of oligonucleotide sequences exhibits some chemoselectivity for different amino acid side chains auguring well for higher chemoselectivity when sequence variability is combinatorially explored in future studies. If this chemistry was realized with glutamic or aspartic acid, it is likely that the prebiotic activating agent would also activate their side chains to nucleophilic attack. Whether these amino acids are relevant at the origins of translation would have to take this into account.
Yields of the intermediate phosphoramidate-ester 4 were greatly improved when the reaction was performed under eutectic conditions as was stereoselectivity, but we do not know why. The rates of ester formation and hydrolysis and competing direct EDC hydration are all likely to be slowed at −16 °C but probably differentially...
The experimental section mentions the use of a compound that was almost certainly not present in prebiotic conditions:
A chemically synthesized RNA-amino acid phosphoramidate (2-Gly, 3′-AGCGAp-Gly-OH, 100 μM) was mixed with ester acceptor RNA strand 3 (3′-ACCUUUCGCU, 100 μM) and activation buffer (made up of water-soluble carbodiimide EDC and imidazole in buffered aqueous solution). While EDC is prebiotically implausible, we use it here as others have done previously as a model-activating agent, allowing the formation of an acyl imidazolide.
EDC, ironically water soluble is a chemical dehydrating agent, but it is possible to imagine many other routes to acy imidazole, some of which may have been prebiotically plausible.
Interesting paper.
Have a nice evening.



