Science
Related: About this forumAlginate-Based Ionic Polymer Composite Derived from Seaweeds for Efficient Iodine Capture
The paper to which I'll briefly refer in this post is this one: Alginate-Based Ionic Polymer Composite Derived from Seaweeds for Efficient Iodine Capture Qian Huang, Jie Fu, Yuan-Hao Wang, Shuang-Long Wang, Jia-Ying Liu, Shi-Jie Guo, Song Qin, Guo-Hong Tao, and Ling He ACS Sustainable Chemistry & Engineering 2024 12 (8), 3089-3099.
This is about the capture of radioactive iodine, including but not limited to 129I, a slightly radioactive form of iodine with a half life of over 15 million years.
I discussed this isotope, 129I, it's release by French Nuclear Fuel Reprocessing, and its appearance in the Mississippi River some 17 years ago on a website where I was ultimately banned for telling the truth: Radioactive Isotopes from French Commercial Nuclear Fuel Found In Mississippi River.
(If one is going to get banned somewhere, there's no better way to do that than telling the truth, an inconvenient truth as they say.)
I'm perfectly OK, if one must know, with the release of 129I to the environment, although I'm rather fond, for economic and technical reasons, with retaining it for use. It is, however, radioactive.
Anyway, the authors of this paper represent that 129I is problematic, and although I disagree, I do favor the use of radioiodine in settings where highly radioactive isotopes are present 131I notably.
From the introduction to the paper:
So far, adsorption has become a widely used method of iodine capture for its versatility, effectiveness, and maneuverability. Common porous solid adsorbents include silver-containing materials, (7) metal–organic frameworks (MOFs), (8−10) conjugated microporous polymers, (11−13) covalent organic frameworks (COFs), (14−17) porous aromatic frameworks, (18,19) etc. Furthermore, ionic liquids (ILs) and deep eutectic solvents (DESs) have been used to efficiently capture and store I2 due to the halogen bonding between I2 and halides in ILs/DESs. (20−22) Although most of these adsorbents have considerable iodine adsorption capabilities, they are inevitably synthesized by expensive monomers and catalysts, complex preparation processes, or harsh conditions, which are not suitable for large-scale applications. Therefore, developing effective iodine adsorbents directly from accessible and low-cost starting materials, especially natural biomass resources, is a feasible strategy.
Seaweeds, mainly macroalgae that can be seen with the naked eye, are classified into three major groups: brown algae (Phaeophyceae), green algae (Chlorophyta), and red algae (Rhodophyta). (23) There are many kinds of seaweeds, and to date, tens of thousands of different seaweeds have been identified. (24) Seaweeds generally reproduce asexually, which allows for fast propagation of the species. Moreover, it has been more than 70 years since seaweeds were shown to be among Earth’s most productive organisms. (25) Due to their rapid regeneration, seaweeds grow in abundance in oceans. The annual macroalgae harvest from wild and cultivated crops was 28.4 million tons in 2014. This is a rise of 43% compared to 2010, when 19.9 million tons of seaweed were harvested. (26) On the other hand, the massive scouring of coastal shores by seaweeds has caused serious economic damage to tourism, aquaculture, and traditional fisheries. The “algal bloom” caused by seaweed flooding occurred in 126 coastal countries globally. (27) Therefore, fast-regenerated and abundant seaweeds may be biomass resources for sustainable development. The industrialization of seaweeds has a history spanning more than 300 years. Whether from aquaculture or algal blooms, seaweeds contain considerable amounts of alginic acid (HAlg). Due to their abundance, renewability, and low cost, alginic acids are widely used in food, pharmaceuticals, and biomaterials. In the laboratory, alginate’s abundance of hydroxyl and carboxyl functional groups makes it an ideal raw material for the preparation of a variety of functional adsorbents...
I'm not entirely sure that there's a "great need" for this reagent under present conditions, for various reasons I favor the fast recycling of highly radioactive nuclear fuel, so such a polymer might prove useful under these conditions. I also favor the production of highly radioactive isotopes of iodine, 128I and 130I as a source of xenon.
As I pointed out 17 years ago, and have yet to change my mind, I don't think that the removal 129I is justified only when there is an economic or serious environmental reason for doing so, and right now there isn't such a reason, health or otherwise. There are more useful and productive ways to spend money to save human lives than worrying about 129I. However the exposure of air to intense radiation has much to recommend it though I don't have time right now to discuss why that is.
Anyway, here's a graphic about the rather simple process utilized to make this iodine capture agent from seaweed:
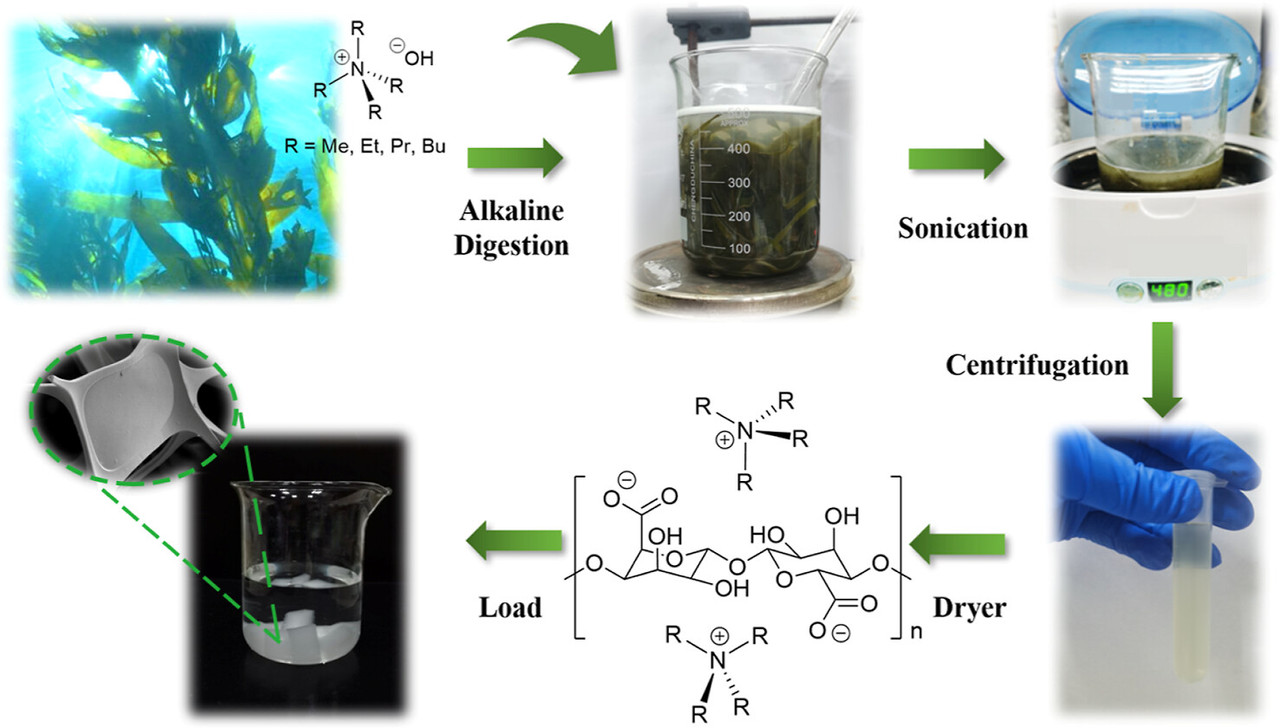
The caption:
Obviously the product sequesters carbon in use but this is unlikely to amount to much since the high energy to mass ratio that makes nuclear energy environmentally superior to all other forms of energy means that not all that much is required.
The authors report that their product is extremely cheap to make, very effective, and recyclable.
I hope you're having a pleasant Sunday afternoon.