Science
Related: About this forumA Useful Tutorial on the Analysis of Polymers.
Over the years, given the limits of techniques available to me, I have struggled at times, with the characterization of polymers. Since the question only comes up every four or five years or so, I always find myself having to relearn the techniques involved.
Polymer chemistry is, of course, important in a vast number of areas, and, most notably, represents a huge environmental problem in the form of now ubiquitous nanoplastics, now showing up in people's brains (this is actually true) literally. Along with mercury and lead poisoning from the continuing and rising use of dangerous fossil fuels, this may account for the elevation of the stupid and evil by the stupid and evil in the United States and elsewhere.
Since I confess that I sometimes drift back and forth through my journal here to remember what I was considering at some point or another, I thought I'd note the following paper, which is mildly unusual in the primary scientific literature (but certainly not unknown), billing itself as a "tutorial:"
Polymer Molecular Weight Distributions: A Tutorial on Transformations between Number Density, Mass Density, and Linear/Logarithmic Axes Ziqiu Chen, Changhae Andrew Kim, Yi-Yu Wang, Aaron D. Sadow, Anne M. LaPointe, and Baron Peters Industrial & Engineering Chemistry Research 2025 64 (7), 3695-3703.
Some text:
The reactants and products in polymer upcycling can be analyzed by various measurement techniques, such as gas chromatography (GC), gel permeation chromatography (GPC), etc., according to their carbon number and phase. (5) In this process, molecular weight distribution (MWD), also known as molar mass distribution, is often used for polymer characterization. (6−8) The evolution of the MWD over time can be used to provide insights into the polymerization and depolymerization mechanism. (9−11) Polymer MWDs can be represented in several different ways. The simplest representation gives the number or concentration of polymers with lengths between n and n + dn as ρ
The ability to interconvert between MWD representations is essential for drawing accurate conclusions about mechanisms (13) and for comparing results from different experiments on an equal footing. For example, an MWD that appears to be monotonically decreasing in one representation can appear to have a focused maximum in another representation. The alternative representations have been discussed in previous works on polymer MWDs, (14,15) and different MWD representations were widely used in the field of polymer reactions. (16−18)
This paper aims (i) to present general experimental considerations for studying products with a wide range of molecular weights, thermal properties and solubilities, since this is often crucial for comprehensive assessment of depolymerization reactions, (ii) to provide a tutorial on the interpretation of MWDs in different representations, (iii) to collect the conversion formulas between various representations into one article, and (iv) to show how the different representations are used in different experimental and theoretical analyses...
A number of techniques are summarized in the paper; the only one to which I've ever had access is GPC/RI (gel permeation HPLC using a refractive index detector), although I did try to convince a guy in our lab to utilize a mass spec technique with which he had only limited success, and at another time, we once quantified a PEG polymer in blood by LC/MS/MS utilizing the monomer generated by ESI (electrospray ionization, a common mass spec technology. This technology is not mentioned in the paper, nor, interestingly DLS, dynamic light scattering except peripherally; which at various times, I tried to convince my management to explore, albeit unsuccessfully.)
There is some very cool mathematics in the paper - many beyond the analytical results on which I have focused in my career - unfortunately I won't have time to discuss at them, but perhaps we can look at the pictures to get some insight to some techniques:
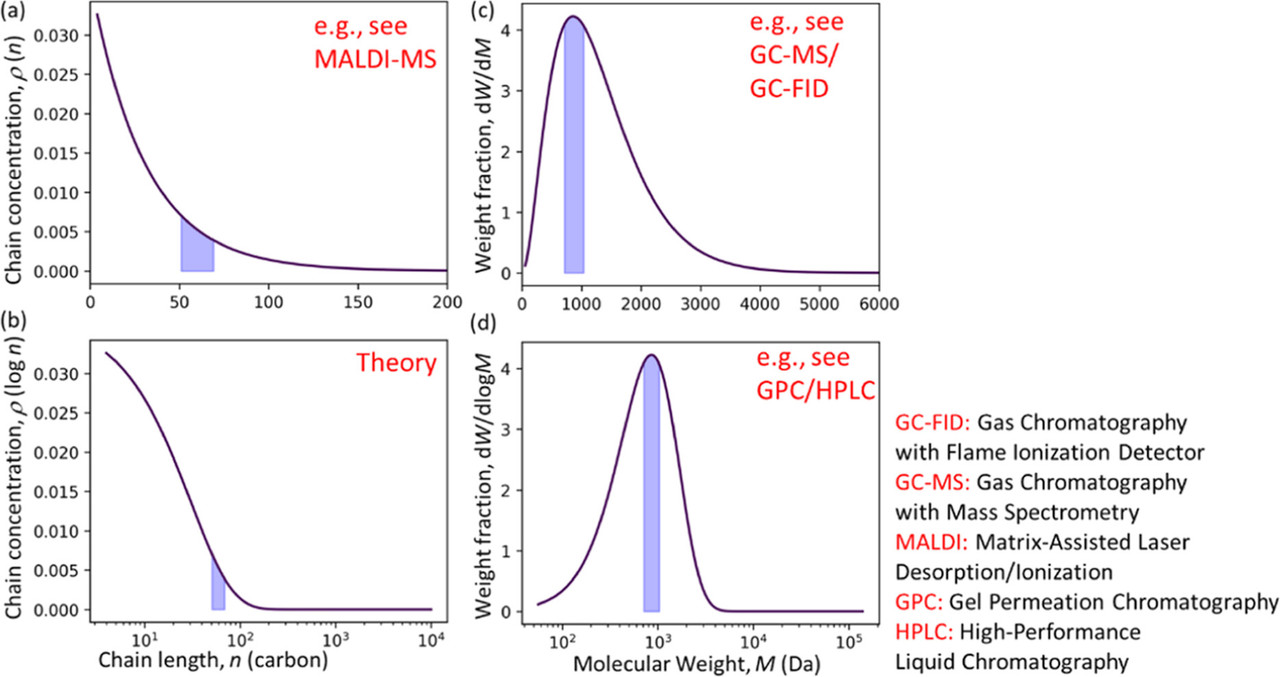
The caption:
A schematic of techniques around expected molecular weight distributions:
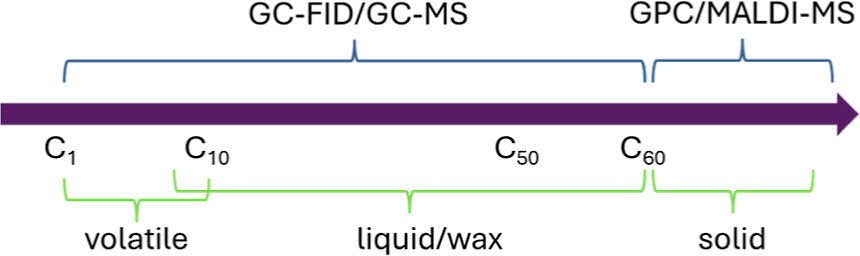
The caption:
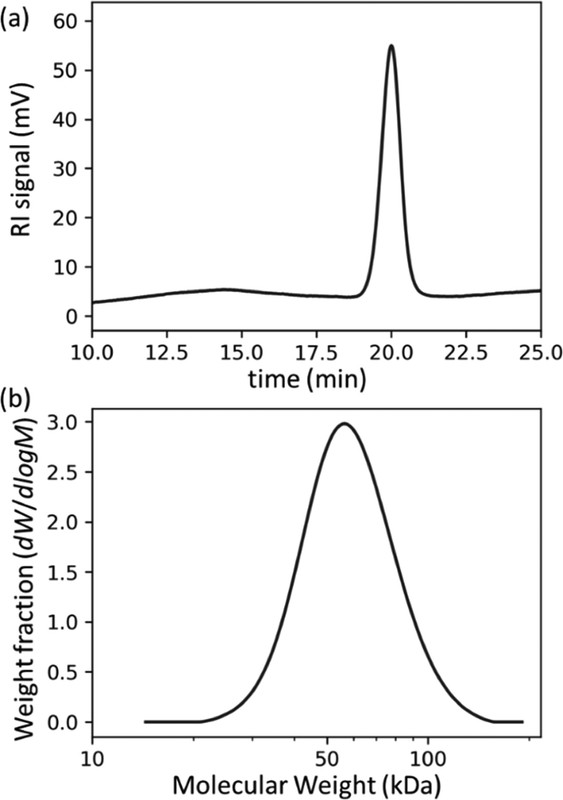
The caption:
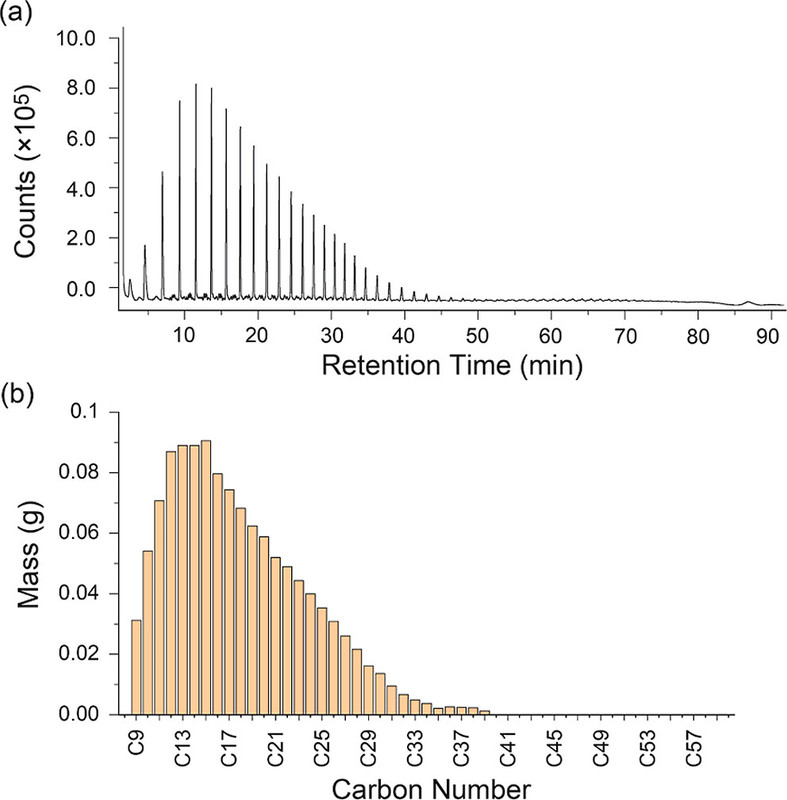
This technique is totally new to me; it seems to involve pyrolysis of a polymer on a GC column, which I can't imagine is good for the column, but I probably am missing something:
A related technology into which I have looked, without convincing management to buy into an instrument, is DSC, differential scanning calorimetry, which can certainly characterize the types of polymers with which one is dealing, useful in environmental settings where polymer types may be unknown.
Anyway, it's a cool paper. I know there are other chemists on DU - a political website that happily has a nice science section - and perhaps, as I have, they have struggled with polymer characterization - and in any case, given the environmental implications of polymer production and use - it's useful to know.
Easterncedar
(6,068 posts)Thanks NNadir.
NNadir
(37,825 posts)It's a great way to learn new things, exposing oneself to what one can't understand.
I recommend it highly. Congratulations to you are in order.